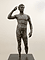|
 |
 |
 |
Grades/Level: High School (9–12)
Subjects: Visual Arts, Science
Time Required: 3–5–Part Lesson
1–3 class periods (plus one week for submerging metal in water)
Author: J. Paul Getty Museum Education Staff
Permissions: 
The lesson plan and downloadable materials on this page are licensed under a Creative Commons Attribution 4.0 International License. |
 |
|
 |
 |
 |
 |
 |
 |
 |
Lesson Overview |
 |
Students study an ancient bronze statue, analyze its pose, and discover how conservators remove and prevent corrosion. They learn that the bronze used to make this sculpture is an alloy of copper and tin with small amounts of antimony, lead, iron, silver, nickel, and cobalt. They use the periodic table to research the chemical formulas of compounds used to make bronze. After learning about oxidation-reduction reactions that occurred in the statue, students speculate about the conservation techniques needed to conserve the bronze sculpture.
|
 |
 |
 |
 |
 |
Learning Objectives |
 |
Students will:
• Observe and understand the changes that occur to metals when submerged in water.
• Analyze the pose of an ancient Greek sculpture.
• Use a periodic table to identify the elements that compose bronze and understand the process by which these elements combine to form bronze.
• Understand the oxidation-reduction reactions that occurred in a statue when it was made and then when it was exposed to seawater.
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
Materials |
 |
• Materials listed in the beginning-level lesson
• Video: Conserving Bronze: The Lamp with Erotes from Vani
• Digital projector with Internet access or a computer lab
|
 |
 |
 |
 |
 |
|
|
 |
 |
 |
 |
 |
Lesson Steps |
 |
1. Complete steps 1–5 of the beginning-level lesson and steps 2–3 of the intermediate-level lesson.
2. Explain to the class that some of the corrosion of Victorious Youth was caused by a chemical reaction between the bronze and the oxygen in the salt water. This chemical reaction resulted in an electron transfer known as oxidation. This means that the metal compound of bronze was combined chemically with the oxygen in the water to create an oxide. In this process, electrons were released at the anode (salt water) and taken up at the cathode (statue).
3. Have the class balance the oxidation-reduction reactions that occurred in the bronze when the statue was submerged in the ocean.
• Electrochemical corrosion of copper alloy leads to the production of cuprous ions. Cuprous ions combine with the chloride in the seawater to form cuprous chloride, a major component of the corrosion layer:
Cu – e →
Answer: Cu – e → Cu+
Cu+ + Cl- →
Answer: Cu+ + Cl- → CuCl
• The statue now contains cuprous chlorides and is recovered and exposed to air. It continues to corrode when cuprous chlorides are combined with moisture and oxygen to hydrolyze and form hydrochloric acid and basic cupric chloride:
4CuCl + 4H2O + O2 →
Answer: 4CuCl + 4H2O + O2 → CuCl2 • 3Cu(OH)2 + 2 HCl
• The hydrochloric acid attacks the uncorroded metal to form more cuprous chloride:
2Cu + 2 HCl →
Answer: 2Cu + 2 HCl → 2CuCl + H2
This process would have continued until no metal remained. Conservators had to scrape off the incrustation layers and find a way to stop the corrosion process.
4. Divide the class into working groups of four students. Explain that to stop the statue from corroding further, the conservators could have chosen among the following methods: electrolytic, electrochemical, sodium-sesquicarbonate, and sodium-sesquicarbonate/vacuum. Students can explore these treatments in detail in Methods of Conserving Archaeological Material from Underwater Sites on the Conservation Research Laboratory website. You may wish to note that the methods mentioned above are used to enhance salt extraction in archaeological metals and that, if the salt extraction is not completed, the reaction outlined in number 3 above would continue to occur.
5. Have groups come up with a combination of methods to conserve the bronze statue. Students should record findings in their journals. Groups will share their findings with the class. Explain that the Getty conservators decided to use the sodium-sesquicarbonate/vacuum method to preserve the artwork. This method guaranteed that all unstable elements would regain stability without any loss to the original bronze or to the artistic qualities of the statue's surface. In addition, the statue is now kept in a humidity- and temperature-controlled room to prevent further corrosion. Compare students' recommendations to the actual treatment.
6. Return to the image of the sculpture and ask students to discuss the artistic qualities of the statue's surface. Which qualities do they think the curators were hoping to retain, and why would this be important?
|
 |
 |
 |
| Victorious Youth, Greek, 300–100 B.C. |
 |
|
 |
 |
 |
 |
 |
 |
 |
Standards Addressed |
 |
Common Core Standards for English Language Arts
Grades 9–12
SPEAKING AND LISTENING
Comprehension and Collaboration
1. Prepare for and participate effectively in a range of conversations and collaborations with diverse partners, building on others' ideas and expressing their own clearly and persuasively.
2. Integrate and evaluate information presented in diverse media and formats, including visually, quantitatively, and orally.
READING
Integration of Knowledge and Ideas
7. Integrate and evaluate content presented in diverse media and formats, including visually and quantitatively, as well as in words.
WRITING
Text Types and Purposes
2. Write informative/explanatory texts to examine and convey complex ideas and information clearly and accurately through the effective selection, organization, and analysis of content.
Research to Build and Present Knowledge
7. Conduct short as well as more sustained research projects based on focused questions, demonstrating understanding of the subject under investigation.
8. Gather relevant information from multiple print and digital sources, assess the credibility and accuracy of each source, and integrate the information while avoiding plagiarism.
For more national and California state standards for this curriculum, refer to the charts found in the links at the top right of this page. |
 |

|
 |
 |
 |