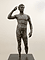|
 |
 |
 |
Grades/Level: Middle School (6–8)
Subjects: Visual Arts, Science
Time Required: 3–5–Part Lesson
1–3 class periods (plus one week for submerging metal in water)
Author: J. Paul Getty Museum Education Staff
Permissions: 
The lesson plan and downloadable materials on this page are licensed under a Creative Commons Attribution 4.0 International License. |
 |
|
 |
 |
 |
 |
 |
 |
 |
Lesson Overview |
 |
Students study an ancient bronze statue, analyze its pose, and discover how conservators remove and prevent corrosion. They learn that the bronze used to make this sculpture is an alloy of copper and tin with small amounts of other elements. They use the periodic table to research the chemical formulas of compounds used to make bronze. Students compare conservation techniques in two ancient bronze objects.
|
 |
 |
 |
 |
 |
Learning Objectives |
 |
Students will:
• Observe and understand the changes that occur to metals when they are submerged in water.
• Analyze the pose of an ancient Greek sculpture.
• Use a periodic table to identify the elements that compose bronze and understand the process by which these elements combine to form bronze.
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
Materials |
 |
• Materials listed in the beginning-level lesson
• Video: Conserving Bronze: The Lamp with Erotes from Vani
|
 |
 |
 |
 |
 |
|
|
 |
 |
 |
 |
 |
Lesson Steps |
 |
Note: This activity assumes that students have some prior knowledge of the periodic table and have had some exposure to basic chemistry.
1. Complete steps 1–5 of the beginning-level lesson and share information about the featured work of art.
2. Explain to students that bronze is a substance usually made of the elements copper and tin. The statue Victorious Youth was made of bronze containing copper, tin, and small amounts of antimony, lead, iron, silver, nickel, and cobalt. It also had patinas on its surface that contained copper and tin. Have students identify the elements copper and tin on the periodic table. Ask students to identify the characteristics of these elements based on their placement in the periodic table. Instruct students to research and record the electrical- and thermal-conductive properties of the two metals. Explain that the warm temperature of the seawater in which the statue was found sped up the corrosion of the metal. Ask students to identify which of the metals in the statue would be most affected by heat based on its thermal-conductive properties. Tell students that the combination of the corrosion layer and incrustations of sea life might have formed a protective exterior cocoon that preserved the interior and allowed the statue to last for over two thousand years.
3. Explain to the class that the tin and copper in the statue were compounds or alloys that were combined to make another compound, bronze. The tin was really tin oxide (SnO2), and the copper was really cuprous oxide (CuO2). Do not share the chemical formulas with the class. Instead, students should use a periodic table to identify the various elements and write the chemical formula for each compound. Explain that the two metals were melted and mixed together to form bronze. Bronze is created when high temperatures release the oxygen. Explain that bronze has its own properties that are distinct from those of copper and tin. Bronze oxidizes very slowly when exposed to air. It is less malleable than copper but has the same conductivity. Overall it has little ductility.
4. Have students view the video Conserving Bronze: The Lamp with Erotes from Vani. Ask students to describe the similarities and differences between the conservation techniques used to clean and restore Victorious Youth and the techniques used for the lamp in the video.
|
 |
 |
 |
| Victorious Youth, Greek, 300–100 B.C. |
 |
|
 |
 |
 |
 |
 |
 |
 |
Standards Addressed |
 |
Common Core Standards for English Language Arts
Grades 6–8
SPEAKING AND LISTENING
Comprehension and Collaboration
1. Prepare for and participate effectively in a range of conversations and collaborations with diverse partners, building on others' ideas and expressing their own clearly and persuasively.
WRITING
Research to Build and Present Knowledge
8. Gather relevant information from multiple print and digital sources, assess the credibility and accuracy of each source, and integrate the information while avoiding plagiarism.
For more national and California state standards for this curriculum, refer to the charts found in the links at the top right of this page. |
 |

|
 |
 |
 |